#293 A new indwelling urethral catheter (amSafeX)… Dr. Anil Mandhani
This is one of the 2023 KS International Innovation Awards videos selected for inclusion in the Vattikuti Foundation – ORSI Humans on the Cutting Edge of Robotic Surgery Conference, October 6, 7 & 8, 2023 in Ghent, Belgium. Posting does not imply that is has been selected as a Finalist, just that the content will be discussed at the Conference.
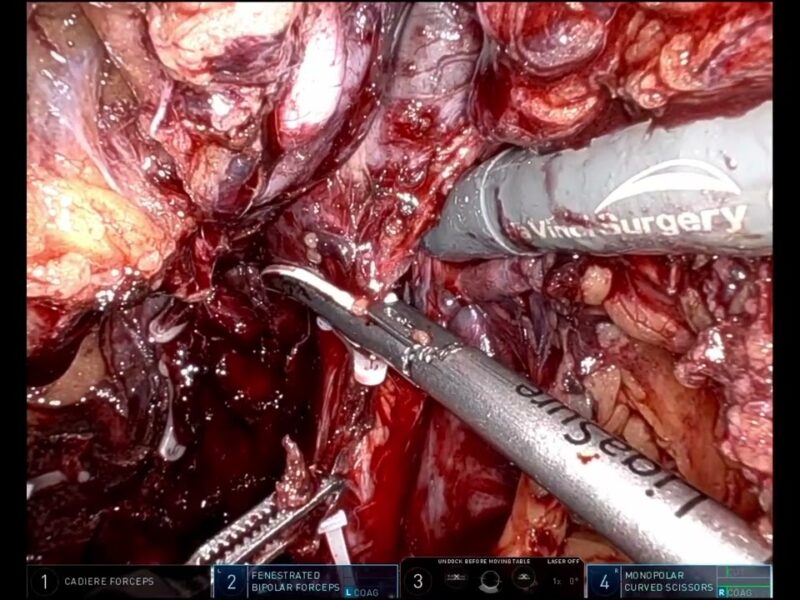
From the entry: Full Title: A new indwelling urethral catheter (amSafeX) to verify the positioning of its balloon within the bladder during inflation. Objective:Inserting a Foley catheter for urethral catheterization can lead to numerous iatrogenic injuries in patients, as it is difficult to ensure that the balloon is in the bladder during inflation.This study aimed to assess the safety of a new catheter, that confirms the presence of the balloon in the bladder during inflation.
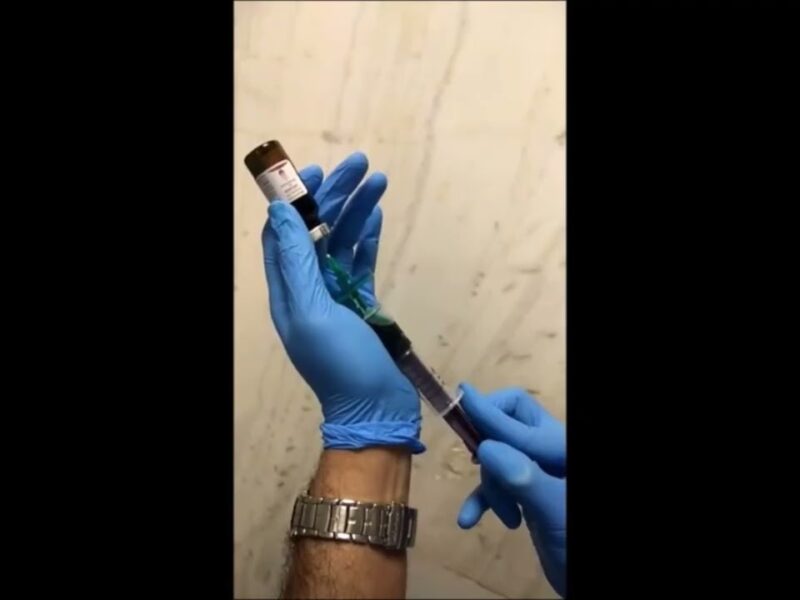
Methods: One hundred male and 25 female patients were catheterized using the amSafeX catheter by a nurse or trainee doctor for various reasons. The catheter featured two draining eyes, with an additional eye proximal to the balloon and the usual distal eye blocked with an internal obturator attached to the string coming out of the drainage port. Standard instructions were given to follow the iSIP protocol of urethral catheterization: insert the catheter, see urine at the catheter outlet, inflate the balloon with 10 ccs of water, and pull out the obturator. Data were collected and analyzed for the safety and efficacy of catheterization.
Result: From Jan 2022 to Oct 2022, none of the 125 patients had any adverse events during catheterization, and the obturator could be removed easily in all procedures. There were no incidents of peri-catheter leakage or urethral injury recorded during catheterization. The median duration of catheterization was 5 (3-12) days.
Conclusion:The amSafeX catheter ensures inflation of the balloon within the bladder, potentially eliminating the possibility of urethral injury.
See more at: https://vattikutifoundation.com/videos/
Date
August 15, 2020